COVID-19 vaccine newsletter - April 13, 2021
Johnson & Johnson vaccine “on pause”
All vaccine providers in Olmsted County will pause use of the Johnson & Johnson (J&J) vaccine starting immediately, following guidance from the FDA/CDC. Use of the J&J vaccine will be put on hold until we receive further recommendations from state and federal partners about how best to move forward. Safety is the highest priority when it comes to all COVID-19 vaccines.
This action is being taken out of an abundance of caution based on the appearance of a rare but serious side effect including brain blood clots (CVST) combined with low platelet counts in six patients, all women under 50. The CDC’s Advisory Committee on Immunization Practices (ACIP) will review these cases in the days ahead and recommend guidance going forward.
If you received the J&J vaccine and developed severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination, please contact your health care provider.
Need proof of vaccination?
At this time, proof of COVID-19 vaccination is not needed; however, many people received a COVID-19 vaccine card when receiving their vaccination. The card is for your personal records – you don’t need to worry if you have lost, misplaced, or forgotten to enter the information on the card. Should you need your vaccination information, you have a couple of options:
- If you received your vaccine at an Olmsted County Public Health Services (OCPHS) clinic: Send an email to healthweb@co.olmsted.mn.us and request your vaccination information. Please include your full name in the email. A screenshot of your vaccine record at OCPHS will be sent within a few days.
- If you received your vaccine at a medical provider or pharmacy: Contact your provider directly and ask to have a copy of your vaccine record emailed, mailed, or available for pickup from the provider.
- If you received your vaccine at OCPHS/Provider/Pharmacy: Request a copy of vaccine record from the Minnesota Immunization Information Connection (MIIC). Your request may take 5-8 business days to process.
COVID-19: Reasonable accommodations
The following information comes from the Minnesota Department of Human Rights.
The Minnesota Work from Home Order expires on April 15, 2021, at which time employers may request employees return to workplaces. Employers are encouraged to allow employees who can work from home to do so.
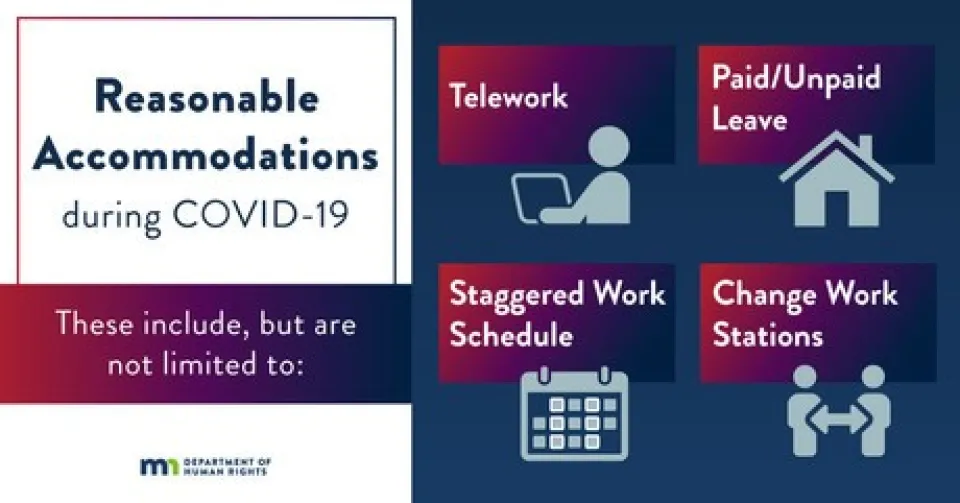
If an employee has a disability that affects their risk of contracting COVID-19 or being harmed if they do contract the virus (such as diabetes, a compromised immune system, or pregnancy), they have the right to request a reasonable accommodation from their employer. Reasonable accommodations include, but are not limited to, telework, paid/unpaid leave, a staggered work schedule, and changing workstations.
COVID-19 education and outreach in Olmsted County
We continue to work with our local FQHC (Community Health Services), medical partners (Mayo Clinic and Rochester Clinic) and key community partners (including IMAA, Rochester Healthy Community Partnership, Diversity Council, local churches and many more) to provide important and accurate information about COVID-19 disease and vaccines to our cultural communities. We are using a community connector model that uses well-known, respected community members and influencers, to co-create and spread messages through town hall meetings, social media, and other avenues to help build vaccine confidence and assist with vaccination clinics. Watch this three-minute video to learn more.
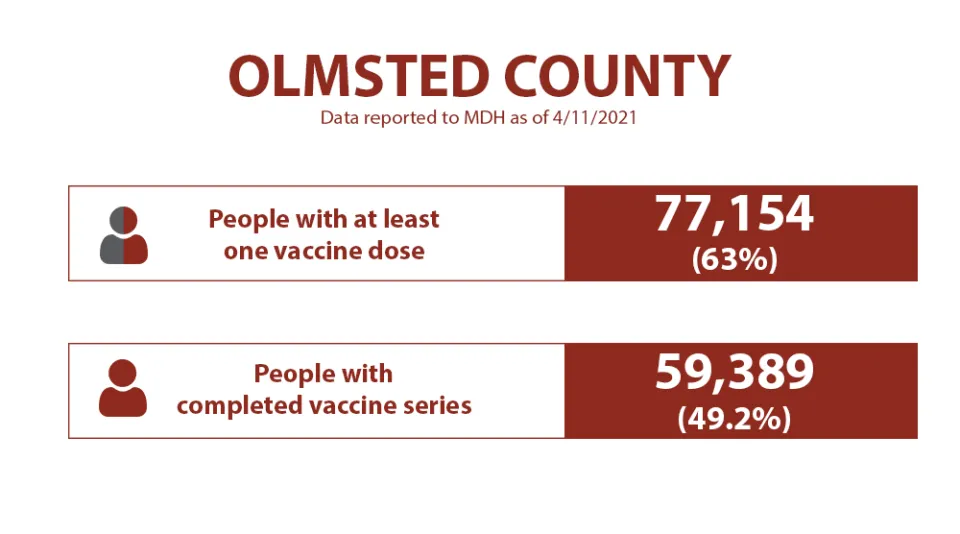
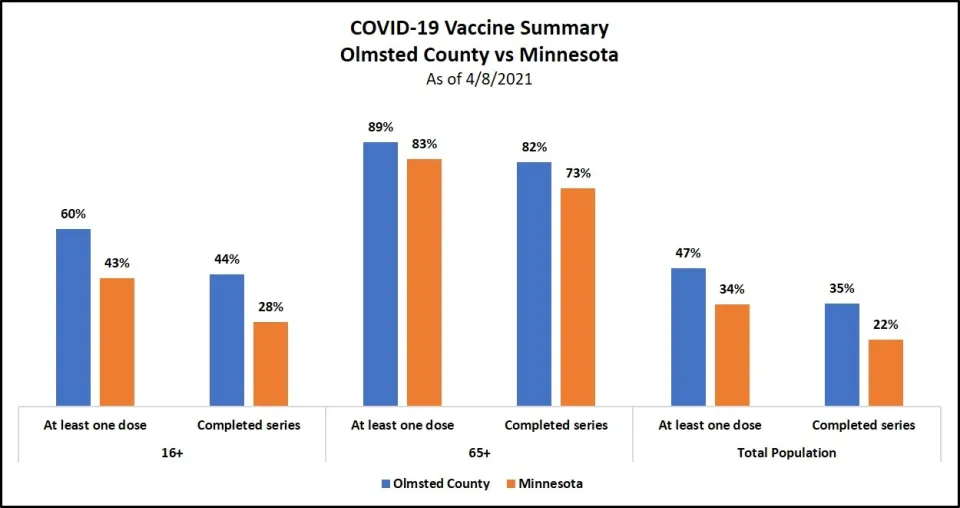
Vaccination status
Olmsted County continues to make steady progress and is ahead of the state in vaccinations for all age groups.
Vaccine updates
Olmsted County Public Health Services (OCPHS)
OCPHS continues to prioritize essential workers and employers per the governor’s order and giving priority to businesses who have completed the COVID-19 Vaccination Planning for Business Form. OCPHS is also working with local school districts to help us reach parents of children ages 16-17 and schedule vaccinations. To date, OCPHS has vaccinated nearly 15,000 individuals. When clinics are not filled with individuals from our business community, we offer the vaccine to people who have registered in advance. Vaccine clinics are by appointment only and are first-come, first-served. The Olmsted County website will be updated with new dates and times based on OCPHS's vaccination allocation. Individuals should check the website daily for changes and updates.
Mayo Clinic
Mayo Clinic will continue to follow a balanced approach, in accordance with state directives, as it continues to prioritize vaccination for patients. Mayo will send vaccine appointment invitations to patients with underlying conditions and as vaccine supply allows, increase vaccination opportunities for Minnesota residents who are 16 and older.
Mayo Clinic patients who have a Patient Online Services account are invited to check the New Appointments section to see if appointments are available. If you don't have a Patient Online Services account, call Mayo Clinic Customer Assistance at 877-858-0398 (toll-free) to create one. If no appointments are available, check back as appointments are released as vaccine is received, or check the VaccineFinder page at the Centers for Disease Control and Prevention, or the MDH Vaccine Connector, which lists vaccination location information.
It may take several weeks to accommodate all patients eligible to receive vaccine in Rochester. If you find an earlier opportunity through another provider who has vaccine on hand, you should take it.
Olmsted Medical Center (OMC)
OMC will be vaccinating the following groups of patients:
- Age 16+ with one or more of the following underlying conditions: Sickle cell disease, Down syndrome, Chronic Kidney Disease (CKD), Chronic Obstructive Pulmonary Disease (COPD), cancer, heart conditions, immunocompromised, obesity, Diabetes Type 1 or 2, and pregnancy.
- Age 50-64, regardless of current health status.
OMC will continue to use a randomized process for determining who will be offered a time slot to be vaccinated.
OMC will reach out to patients in the following ways:
- If you have an OMC MyChart patient portal account, a message will be sent to you through the portal.
- If you have email notifications for messages turned on, the email will read “A new COVID-19 vaccine scheduling ticket available in OMC MyChart.”
- If you have questions about OMC MyChart, you can call 507-287-2780 (8 a.m. to 5 p.m., Monday through Friday).
- If you do not have an OMC MyChart account, you will be contacted by phone or text message.
Please do not call your care provider about getting the vaccine, they will contact you.
Education / information
Vaccine safety
The following information comes from the Centers for Disease Control and Prevention.
Anaphylaxis after COVID-19 vaccination is rare. If this occurs, vaccination providers can effectively and immediately treat the reaction.
CDC and FDA scientists have evaluated reports from people who experienced a type of severe allergic reaction—anaphylaxis—after getting a COVID-19 vaccine. Anaphylaxis after COVID-19 vaccination is rare and occurred in approximately 2 to 5 people per million vaccinated in the United States based on events reported to the Vaccine Adverse Event Reporting System (VAERS). This kind of allergic reaction almost always occurs within 30 minutes after vaccination. Fortunately, vaccination providers have medicines available to effectively and immediately treat patients who experience anaphylaxis following vaccination. Learn more about COVID-19 vaccines and allergic reactions.
The CDC reports that 167 million doses of COVID-19 vaccines were administered in the United States from December 14, 2020, through April 5, 2021. During this time, VAERS received 2,794 reports of death (0.00167%) among people who received a COVID-19 vaccine.
CDC and FDA physicians review each report of death as soon as notified and CDC requests medical records to further assess reports. A review of available clinical information including death certificates, autopsy, and medical records revealed no evidence that vaccination contributed to patient deaths. CDC and FDA will continue to investigate reports of adverse events, including deaths, reported to VAERS.
For more information, view the CDC website.
